Drawing blood, spinning the centrifuge, and then not getting a good PRF clot/membrane can certainly be frustrating. In this post, we will review the potential reasons for why this might happen.
What is a PRF Clot?
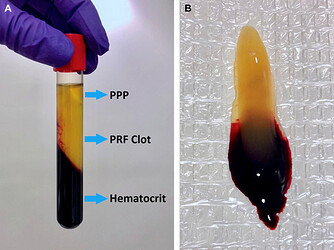
A PRF clot (platelet‑rich fibrin clot) is an autologous fibrin biomaterial produced by rapidly drawing a small amount of venous blood into a vacuum tube and centrifuging it without any anticoagulant. The process concentrates most of the platelets (about 97 %) and more than half of the leukocytes into a dense, three‑dimensional fibrin network that also contains cytokines, growth‑factor‑rich glycoproteins (such as thrombospondin) and other bioactive molecules. After centrifugation, the fibrinogen concentrates in the middle (and upper) part of the tube (see picture below), forming the PRF clot there. This clot functions as a natural scaffold that promotes cell migration, attachment, proliferation and tissue regeneration in dental, maxillofacial and other surgical applications.
Quick Summary for PRF Clot Issues
Most clot problems stem from an incorrect technique, and have nothing to do with the centrifuge used. Unless you have had the centrifuge for at least 2 years, the PRF Clot is almost certainly not a Centrifuge Issue. In fact, Miron et al. has actually verified this in a paper (1), stating:
The PRF centrifugation device had little effect on the final size outcomes of PRF membranes (~ 15% differences between various fixed-angle centrifuges) However, the differences in the PRF clots produced in the different tubes had a marked and pronounced effect on the final size outcomes of PRF tubes.
The possible reasons for not getting good clot are: allowing the tubes to sit uncapped after the run, slow blood draw, incorrect tube type or faulty tubes, improper proper balancing, and incorrect spin settings . Only consider the centrifuge itself if it’s old (over 2 years) or uncalibrated.
If after considering all of the factors below, you are still having issues getting a good clot, then consider sending back the machine to the manufacturer to check the calibration.
Possible reasons you’re not getting good PRF clots
| Reason | Why it matters | What to do |
|---|---|---|
| Tubes not allowed to “breathe” after spin | After the spin the clot finishes forming only when the tube is exposed to air for ~5 min. Keeping the cap on stops the clotting process. | Remove the caps, place tubes in a sterile holder without lids and let them rest ~5 min before removing the clot. |
| Slow blood draw or delayed centrifugation | PRF must be drawn quickly and spun immediately. A slow draw lets the blood begin to clot in the tube, giving a weak, diffuse clot. | Draw the blood fast (do not use a syringe), fill the vacuum tube, and put it in the centrifuge right away. Most offices get best results with 3 tubes (remaining tubes are filled with water to balance). |
| Wrong type of tube | Solid PRF requires glass tubes or silica‑coated red plastic tubes. Plastic or blue tubes do not promote the needed silica‑induced coagulation, leading to poor clots. | Use glass (or silica‑coated red plastic) tubes for solid PRF; use white‑cap or blue tubes only for liquid i‑PRF. |
| Incorrect spin protocol | Using the wrong RPM/RCF or spin time (or changing protocols mid‑run) can prevent proper fibrin formation. | Follow the validated settings for your particular centrifuge (e.g., on the DALI Horizontal it is 2,300 RPM for 8 min for glass tubes, or 2,400 RPM for 14 min. See details below). Adjust only after mastering the basic protocol. |
| Unbalanced tubes | An unbalanced rotor creates vibration, interrupting the spin and compromising clot formation. | Always balance tubes 2‑by‑2; use a water‑filled tube as a counter‑weight when you have an odd number of blood tubes. |
| Patient‑specific factors | Hematocrit, age, gender, and anticoagulant use affect clot size and quality. Some patients may need longer spin cycles (e.g., 18 min). | Consider adjusting spin time for patients with low blood counts or on anticoagulants; otherwise follow the standard protocol.See details below |
| Centrifuge age/calibration | Except when the machine is >2 years old and possibly out‑of‑calibration, the centrifuge itself rarely causes poor clots. | If the centrifuge is >2 years old, have the manufacturer check speed and timer calibration. |
Notes on the Spin Protocol
There are many different PRF protocols and studies are done using different machines. So it can be confusing to know what protocol to use on a particular centrifuge. The most important thing to remember is that the RCF is the number you want to use to determine the proper RPM. The original L-PRF protocol was ~700 RCF max for 12 minutes. A-PRF was approximately ~200 RCF max for 8 minutes (low centrifugation protocol). Both of these protocols were on fixed-angle machines. Miron’s studies on the horizontal machine (2) revealed that: “The best yield of leukocytes was achieved after centrifugation for 8 min utilizing the 700, 1000 and 1200g protocols. Of those the highest concentration was achieved at 700g for 8 min (owing to the reduced total plasma volume).”
On the DDSGadget Horizon 6 Flex Horizontal Centrifuge, the numbers above (given the R-Max of 127), the numbers above would translate as follows:
700 RCF = 2,200 RPM
800 RCF = 2,400 RPM
1,000 RCF = 2,600 RPM
Our 1st setting on the machine, takes into consideration the above potential values, and we set it at
2,300 rpm / 8mn, as a default. If you want a thicker clot, though, you can run a 2nd cycle at 2,300 RPM for another 4 minutes. Alternatively, many users just make a custom setting of 2,400 RPM for 14 minutes (this is the original protocol from years ago before horizontal centrifugation became popular).
Patient Specific Factors
Another factor that is very important to recognize when dealing with the PRF clot is the patient! There is wide variability in results between patients, and this is something highlighted in many PRF studies including that of Miron, cited below. In patients on some sort of anti-coagulant, there is ongoing research (3), but we have seen recommendations of 18 minute spin cycles for these patients.
Interestingly, there was also variability reported between patients. As erythrocytes and other blood components also play a role in coagulation, as well as the hematocrit counts vary between patients and affect the size of PRF membranes We have previously shown that both females and patients above the age of 65 typically have larger membranes as a result of their lower hematocrit counts. Future research is needed to better understand how centrifugation speed and time may be further optimized to result in similar final cell concentrations from patients with different starting blood cell counts. In summary, patients with lower cell blood counts would theoretically require lower centrifugation speeds, and further computational research is likely needed to optimize the protocols. (3)
References for PRF Clot
- Comparative Study Clin Oral Investig. 2020 Mar;24(3):1171-1182. doi: 10.1007/s00784-019-02981-2. Epub 2019 Jul 19. # Comparison of platelet-rich fibrin (PRF) produced using 3 commercially available centrifuges at both high (~ 700 g) and low (~ 200 g) relative centrifugation forces
- Miron, R.J., Chai, J., Fujioka-Kobayashi, M. et al. Evaluation of 24 protocols for the production of platelet-rich fibrin. BMC Oral Health 20 , 310 (2020). Evaluation of 24 protocols for the production of platelet-rich fibrin | BMC Oral Health
-
- Miron RJ, Dham A, Dham U, Zhang Y, Pikos MA, Sculean A. The effect of age, gender, and time between blood draw and start of centrifugation on the size outcomes of platelet-rich fibrin (PRF) membranes. Clin Oral Invest. 2018;23:2179–85.